ERX-1000 for Obesity
ERX-1000, a first-in-class leptin sensitizer, is in development for the treatment of obesity and related diseases.

Identification Of ERX-1000: First-in-class Leptin Sensitizer
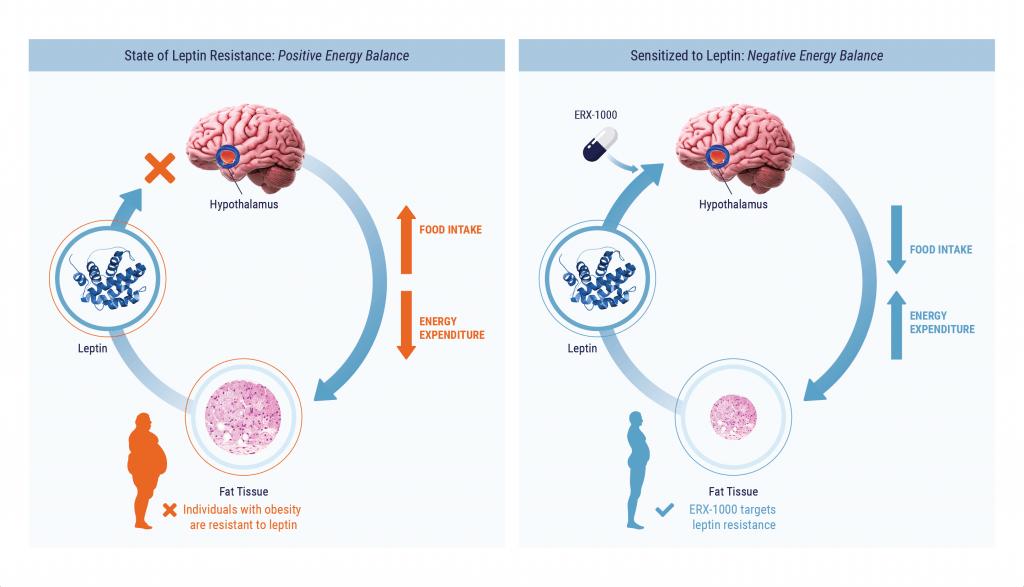
By using a systems-biology approach, ERX scientific co-founder Dr. Umut Ozcan identified ERX-1000, a first-in-class leptin sensitizer. While ERX-1000 is highly effective at reducing appetite and decreasing weight in diet-induced obese mouse models (an experimental model of human obesity), it does not have any effect on leptin-deficient obese mice, obese mice lacking leptin signaling (genetically deficient in leptin or its receptor), or non-obese mice, demonstrating that ERX-1000 is a true leptin sensitizer.
Learn More About Leptin Resistance and Leptin Sensitization
Preclinical Effects of ERX-1000
Energy Intake
Administration of ERX-1000 in a preclinical laboratory setting to diet-induced severely obese mice reduced appetite up to 80% and led to a 45% decrease in bodyweight in 21 days, a level of weight loss that has not been previously observed in any drug in preclinical studies or in animal models of invasive bariatric surgery.
Energy Expenditure
In additional preclinical studies in diet-induced severely obese mice, ERX-1000 demonstrated an effect on the energy metabolism of the body. Under normal conditions, when food intake is restricted, the brain reduces the body’s energy expenditure, which makes it more difficult to maintain weight loss. This has been one of the obstacles in promoting weight loss through diet alone. When the leptin system is functioning properly, leptin suppresses appetite and reduces food intake, while also blocking the brain’s signal to reduce energy expenditure. As a leptin sensitizer, ERX-1000 similarly blocks the messages to reduce energy expenditure in the body, which potentiates its effect on weight loss.
Burning Exclusively Fat
Generally, calorie restriction or diet-induced weight loss is accompanied by some reduction in the body’s muscle mass. However, in preclinical studies of ERX-1000 in diet-induced obese mice, weight loss was achieved through the utilization of fat stores and did not lead to a reduction in muscle mass.

ERX-1000 and Metabolic Health
Improved leptin sensitivity and properly functioning leptin signaling is expected to lead to weight loss and therefore weight-related improvements in metabolic health. ERX-1000 improved glucose tolerance, reduced fasted and non-fasted plasma glucose concentrations, and improved insulin sensitivity in diet-induced obese mice. In addition, Phase 1 data showed early evidence of potential to improve glucose and insulin homeostasis. Moreover, ERX-1000 reduced (almost completely eliminated) hepatic steatosis (accumulated fat in the liver) in diet-induced obese mice and reduced ALT and AST levels after fat loss. These pharmacological effects make ERX-1000 a promising drug candidate not only for the treatment of obesity but potentially for the treatment of obesity-related diseases such as type 2 diabetes.
ERX-1000 Preliminary Clinical Results
The clinical development program for ERX-1000 is designed to support registration for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity in patients with obesity. A first-in-human Phase 1, double-blind, placebo-controlled, single and multiple oral dose study has been conducted in healthy non-obese and obese subjects to determine the safety, tolerability, pharmacokinetics and pharmacodynamics of ERX-1000. Preliminary data from this clinical trial have shown dose responsive weight loss in a 4-week treatment period, thereby yielding initial proof of efficacy for ERX-1000 in obese humans.